Which Base Would Best Separate a Mixture of Below Compounds
Liquid that passes through is called the filtrate. Separating a Mixture of Compounds flashcards containing study terms like What was the mass of the empty evaporating dish.
Separation Of An Unknown Mixture
I am thinking of doing an acid-base extraction but dont know how to since I dont know any physical.
. Heterogeneous Mixtures-These are the type of mixture in which two or more compounds are mixed unevenly or unequally. Mixtures can be separated using various separation methods such filtrationseparating funnelsublimationsimple distillation and paper chromatography. The plan of the experiment is to identify the two compounds.
We add aqueous solutions to our organic compounds so they wash away impurities. To remove inorganic unwanted compounds from what we want we perform a wash. Mixture containing an acidic a basic and a neutral compound is to be separated using acidbase extraction1 The three organic compounds to be separated are cinnamic acid p-toluidine and anisole using dichloromethane as the extraction solvent Figure 1.
TLC is used to determine if certain compounds in the mixture. These reactions are simplified to point out the precipitate in each reaction. There are also chemical methods which are used by rearranging the particles so a certain.
Separate water from Sandy water Evaporation and crystallisation. C 11 Ethyl acetateHexanes D 41. As previously mentioned two techniques are vital to solvent extraction.
Add another drop of water every minute or so to make the chromatogram spread toward the edges of. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles some of which are large enough in size to be captured with a porous materialParticle size can vary considerably given the type of mixture. You will also purify and determine the percent recovery of each compound from the mixture.
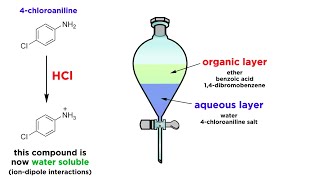
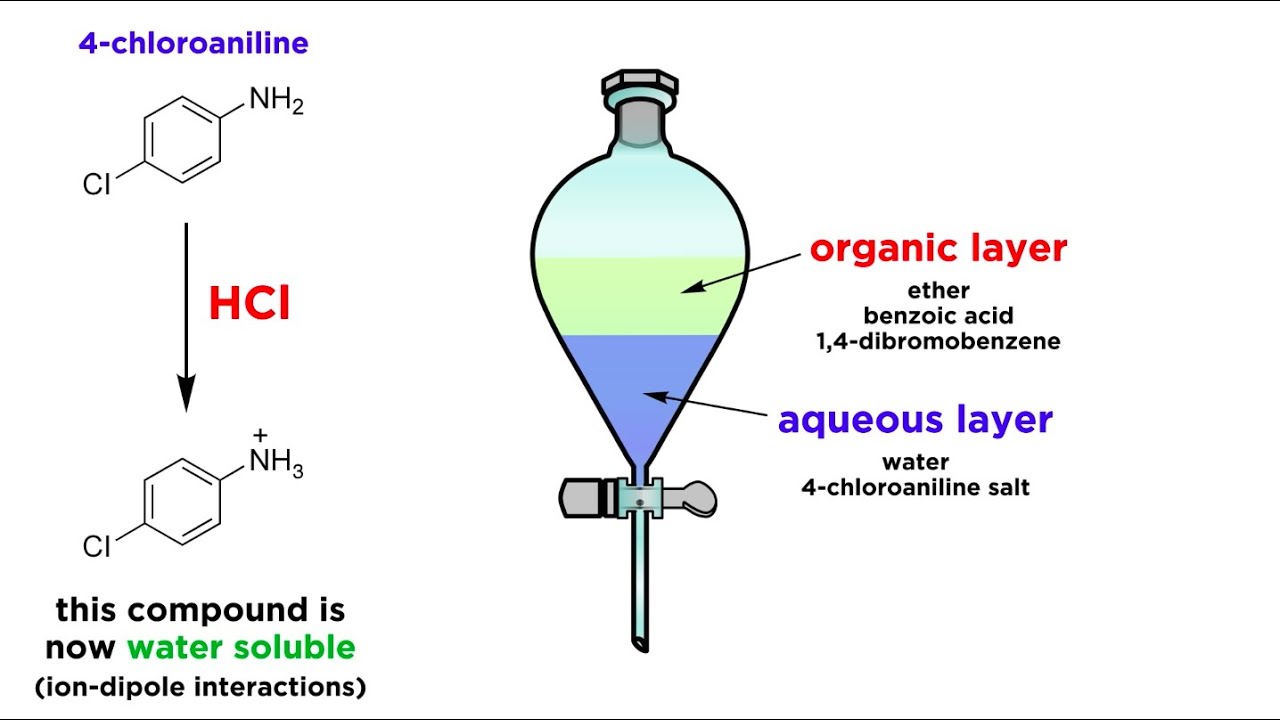
We use different physical characteristics such as boiling point polarity of compounds to separate chemical compounds from mixtures. Ar-COO Na HCl Ar-COOH. Ar-NH 3 Cl NaOH Ar-NH 2.
To remove acids we add bases. Homogeneous Mixtures-These are the types of mixture in which two or more compounds mixed are distributed uniformly throughout the mixture. Sample starting Mixture 1H-NMR Spectra for reference and pre-lab questions Mixture A.
React 1-phenyl-2-propanamine racemic mixture with a chiral acid such as -tartaric acid R RReaction will produce a mixture of diastereomeric salts ie. Record the mass to the nearest 0001 g. For example Air and saline solution.
Triphenylmethane benzoic acid and ethyl 4-aminobenzoate. Memorize flashcards and build a practice test to quiz yourself before your exam. Protect your arms and hands by wearing a long-sleeve lab coat and gloves.
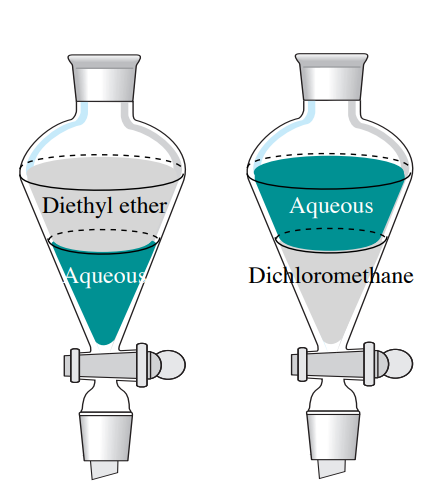
Gently shake the separatory funnel to allow intimate mixing of the solutions and effect extraction of the compound from the organic mixture. Separation of NaCl from sea water is an example for separating chemical compounds from a mixture. Extraction literally taking out by force is a useful technique for separating compounds such as I 2 and KMnO 4 that have different polarities.
KOH to recover the pure enantiomeric amine. The compounds to be separated are treated with a mixture of a polar solvent such as H 2 O. Vacuum filtration and TLC.
If an acid is combined with a base of equal strength the result will most likely be. Triphenylmethane trans- cinnamic acid and ethyl 4-aminobenzoate. A stronger base sodium hydroxide is required to react with the less acidic 2-naphthol.
Conduct this reaction in a fume hood. But sometime we cannot obtain 100 pure compound after separating. Salt from the organic compound which will make it water soluble to separate it from the compounds still soluble in the ether solvent.
Clamp the separatory funnel to a retort stand and allow the mixture to separate into two layers. Though chromatography is a simple technique in principle it remains the most important method for the separation of mixtures into its. R R R and S R RSeparate diastereomers through crystallization.
Remove the stopper and collect the aqueous layer in the 125 mL Erlenmeyer flask labeled hydroxide. A two-component mixture with 2-naphthol in the ether layer can be separated using the stronger base thus allowing 2-naphthol to be extracted from the neutral compound. Compound The neutral compound in your mixture will stay dissolved in the diethyl ether.
Impossible to tell without testing the pH. Answer 1 of 4. Treat salt with strong base eg.
76000 g What was the mass of the evaporating dish plus the powdered mixture. A commonly used method of separating a mixture of organic compounds is known as liquid-liquid extraction. In this experiment you will use extraction techniques to separate a mixture of an organic acid a base and a neutral compound.
Student A wants to separate a mixture of 3 compounds. We could use fractional distillation solvent extraction partial crystallization chromatography and another. Lay the paper disk flat over the top of a beaker.
Transfer the mixture to a 100 mL beaker and dissolve it in 15 mL of diethyl ether. Hello mixture and compund first thing is thr tht all compunds are mixture but all mixture are not compund because a compound is formed when two or more than two different elements combine and formed a new compund with a different chemical and physical properties in comparison. Most reactions of organic compounds require extraction at some stage of product purification.
The methods stated above are all physical methods. Start studying the Quiz 7. Use one of the coloured pens to make a 05 to 1 cm ink spot in the centre of the disk.
The only information given is that the compounds have similar polarities which means they form a homogeneous mixture so to separate them we should use some of the processes of separation of a homogeneous. The process of separating a solid also called a residue from a liquid by means of a porous substance a filter which allows the liquid to pass through but not the solid. Organic acids and bases can be separated from.
Obtain and wear goggles. Chromatography is often used with felttip pens to show that black colour is made up of say blue and yellow. So my primary concern is to separate the binary mixture and purify the individual compounds then the subsequent classification testsNMRIR analysis should be straightforward.
1 the mixture is pored through a funnel lined with a filter paper 2 the filtrate liquid drips through to the filter flask 3 the solid remains in the funnel. Place a drop of water in the centre of the ink spot. For example Oil in water and Sand in water.
For instance stream water is a mixture that contains naturally occurring biological organisms like bacteria viruses and. The student develops the TLC plates but forgot to mark which solvents were used on which plate. One of the most important and time-consuming activities in chemistry involves isolating separating and purifying chemical compounds.
The student uses three different solvents C D and E to try to find a mixture that gives the best separation on a TLC plate coated with silica. What are the types of methods to separate mixtures. Weigh out approximately 10 g of the sample mixture.
Naphthalene benzoic acid and ethyl 4-aminobenzoate.
Separation Of An Unknown Mixture

Separating Components Of A Mixture By Extraction Youtube
No comments for "Which Base Would Best Separate a Mixture of Below Compounds"
Post a Comment